FDA – U.S. Food & Drug Administration:
Field Inspection Application
The FDA requested to develop a tablet-based solution that federal, state and local investigators can effectively use in the field to automate the process of conducting imported food inspections and recording inspection results in FDA business applications.
CASE STUDY
Transforming FDA Field Inspections: Innovation and Digital Transformation
Imagine being an FDA inspector responsible for ensuring the safety of food entering the country. It’s a huge responsibility, but outdated software, endless paperwork, and cumbersome inspection equipment made the job even harder. Add poor communication and long hours, and these inefficiencies further complicate the critical task of protecting public health.
We began by listening to the inspectors’ struggles. We shadowed them, asked questions, and studied their workflow. It became clear that small fixes wouldn’t be enough—a complete digital transformation was needed.
With these insights, my team developed an iPad app that radically improved the inspection process. The app consolidated multiple inspection tools into one device, eliminating the need for inspectors to carry several items.
We introduced digital forms to simplify data entry and real-time communication to help inspectors stay connected with their teams. The app provided instant access to searchable regulations and guidelines. We added voice-to-text for hands-free reporting and integrated a camera for documenting violations. Since connectivity was often unreliable, the app functioned offline to ensure inspections continued without interruption. We also optimized navigation and planning for more efficient travel between sites.
Great design reduces stress and increases satisfaction. This project reimagined the process to align with inspectors’ needs, creating a solution that was not only functional but made their jobs easier. It highlights the power of innovation and human-centered design, enabling inspectors to work more efficiently while enhancing public safety.
KEY OUTCOMES:
• 27% reduction in inspection times
• 22% increase in weekly inspections
• 18% reduction in annual operational costs
• 39% decrease in error rates during inspections
• 52% reduction in missed violations
• 84% in improved job satisfaction
Client: FDA – U.S. Food & Drug Administration
Project: Imported Food Field Inspection Application
Platform: iOS native app – iPad
Role: Senior UX Designer
Project Team
Design Team: Senior UX Designer (myself), 2 UX Designers, Engineering Lead, Project Manager
Client Team: Stakeholders, Product Owner, Engineering Lead, Engineering Team
Time Frame:: 6 Months
Context
Problem
- Inspectors are required to conduct highly detailed inspections that involve thorough documentation and collecting various samples from the imported food items.
- The inspection process is complicated by the use of multiple devices, tight schedules, and back-to-back inspections at different locations.
- Environmental challenges such as varying inspection site sizes, limited wireless connectivity, and technology constraints provide challenges during the inspection process.
Objective
Research, strategize and design an end-to-end mobile solution for Food Inspectors to efficiently utilize in the field.
This solution is intended to:
- Simplify the process of inspecting imported foods and integrate the documentation of inspection results into an easy-to-use application.
- Enhance the efficiency of the inspection procedure for a faster and more streamlined operation.
- Aim to reduce the time taken for inspections by 27% relative to previous practices.
STAGE 1: EMPATHIZE
Tasks
- Review stakeholder requirements
- Educated on inspection training and FDA operating procedures.
- Create a Problems and Challenges matrix.
- Conduct interviews with Stakeholders and Field Inspectors.
- Perform a product evaluation.
- Attended and shadowed Field Inspections with FDA inspectors.
Client Requirements, Inspection Training & FDA Operations
- Discussed and assessed requirements in collaboration with the core team at the FDA.
- Educated in food inspection techniques and import procedures.
- Collected insights on FDA operations.

Problems & Challenges
Created a Problems & Challenges Matrix addressing the current inspection process.
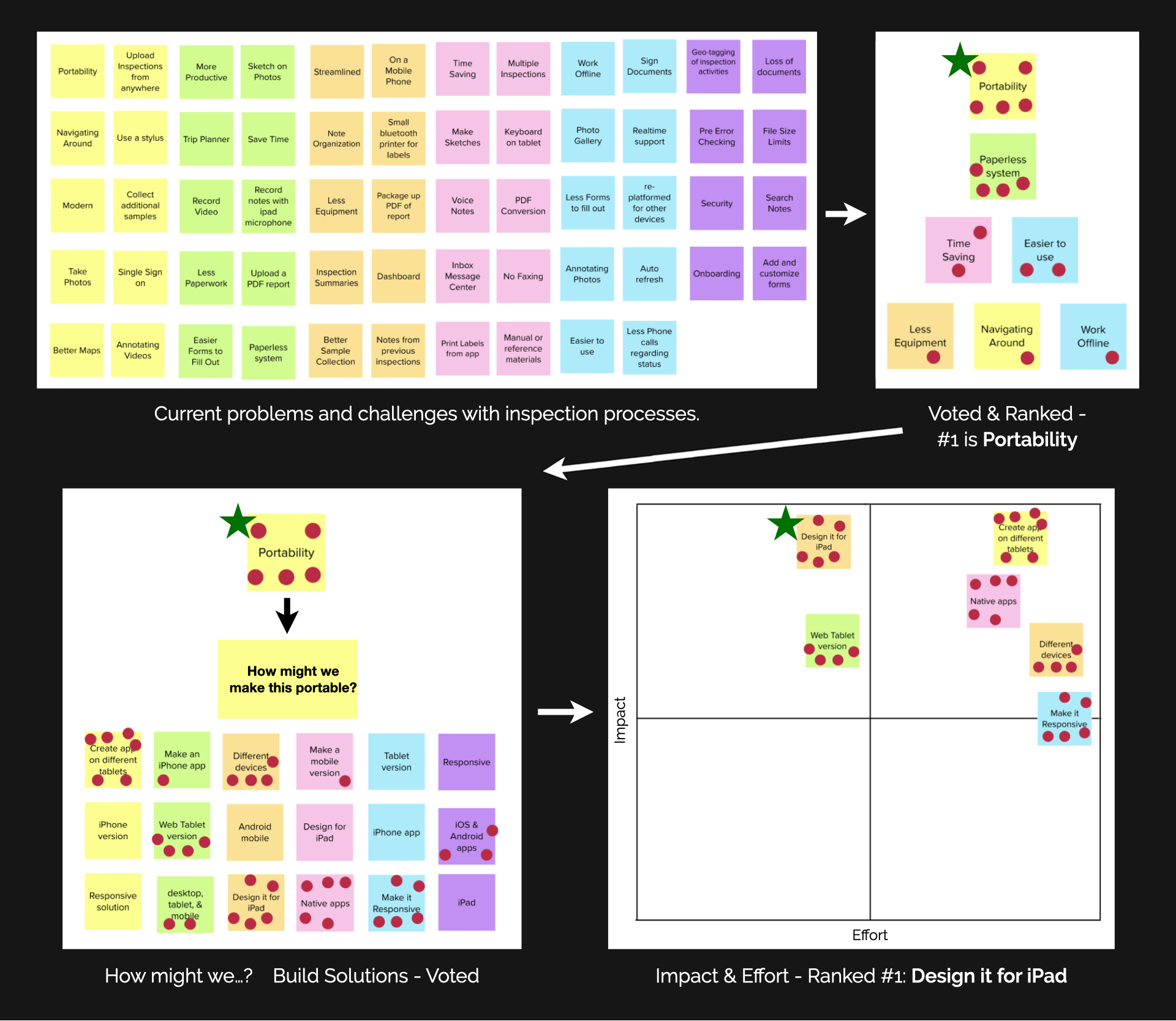
Key Findings & Next Steps
- Review the existing desktop version to adapt it for iPad compatibility.
- Explore iPad features that can utilized to enhance inspection tasks.
- Identify equipment that can be replaced with iPad features.
- Develop versatile methods for capturing information and notes on the iPad.
Interviews
Key Findings
- Inspectors are required to carry a substantial amount of equipment for every assignment.
- Difficulties in filling out forms, retrieving information, and operating offline because of inconsistent connectivity.
- Extended working hours necessitated by frequent phone interactions with the back office and the submission of reports.
- Improve inspector engagement and productivity by maintaining their focus and efficiency during tasks.
- Encourage inspectors to take more initiative and act decisively during and post-inspections.
- Simplify the process of accessing information, setting goals, communicating, and employing technology for inspectors.
Product Evaluation
Reviewed the current desktop inspection application against standard usability principles known as heuristics.
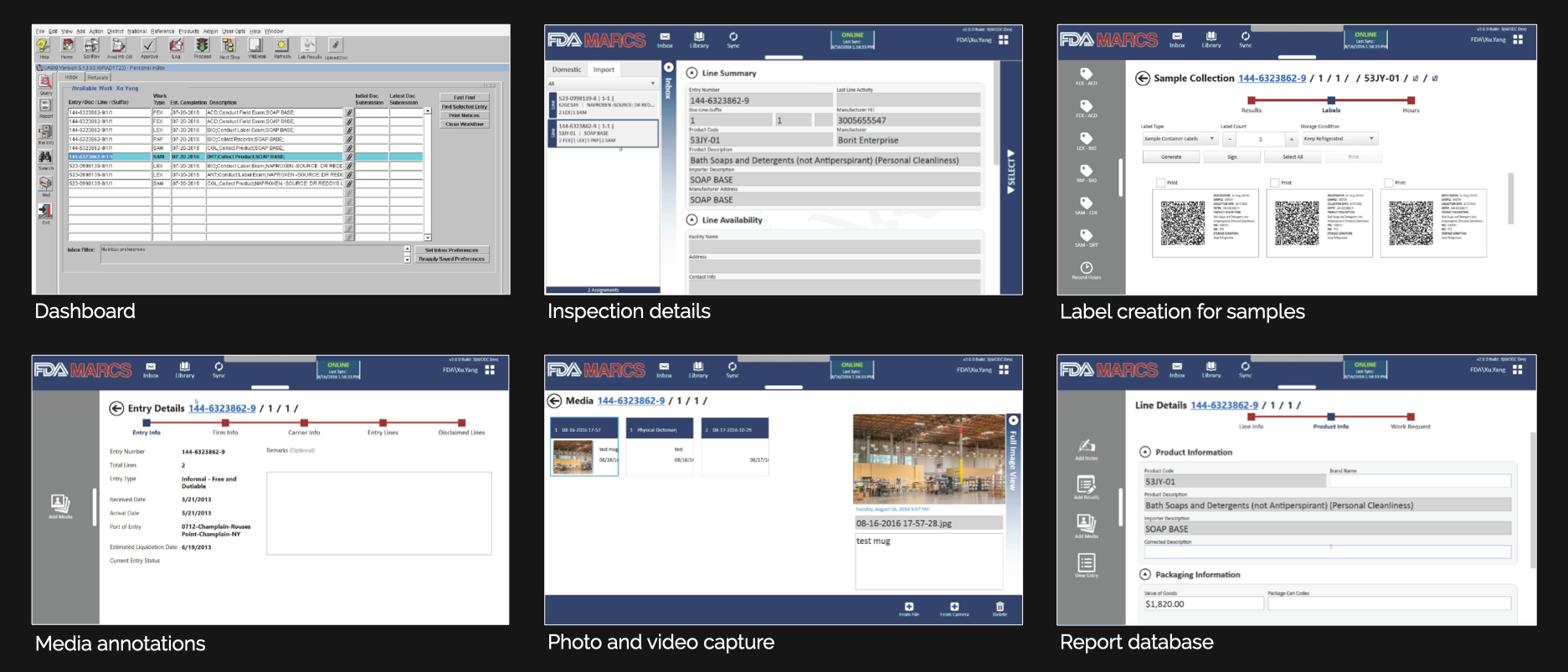
Key Findings
- The software’s user interface is not intuitive, featuring an unclear navigation system and an excessive number of screens, complicating the overall process.
- Information within the software is disorganized, making it challenging to access and locate.
- The process for submitting inspection reports is inefficient, requiring too many manual steps.
- The software is constrained to desktop use, lacking both mobile and web versions, and its design is outdated, failing to align with modern aesthetics.
- Connectivity issues and technological limitations frequently disrupt the software’s functionality, further compounded by the absence of offline support.
Field Study


Key Findings
Technological Improvements: Upgrade outdated software to include intuitive interfaces, mobile, and offline capabilities. Simplify processes with automation, reducing paperwork and enhancing connectivity.
Equipment Management: Reduce the burden of carrying extensive equipment and manage personal expenses for essential work tools.
Time Management: Streamline inspection travel and time management, tackling delays in report approvals and extended work hours.
Team Dynamics: Improve team reliability and communication, addressing punctuality issues and enhancing workflow through proactive strategies.
STAGE 1: EMPATHIZE – Key Findings Overview
The need for significant software upgrades, equipment optimization, and workflow enhancements to improve FDA inspectors’ efficiency and productivity.
By addressing technological limitations, simplifying processes, and improving team dynamics, inspectors can be better equipped to perform their duties effectively, reducing the physical and administrative burdens they currently face.
STAGE 2: CONCEPTUALIZE
Tasks
- Develop User Personas
- Design a Comprehensive Journey Map
- Conduct Foundational & Exploratory Research
User Personas

Key Findings
Both inspectors share common challenges with technology and process inefficiencies, and they express a need for modernized systems that would allow for more streamlined, effective work and better communication. Despite their frustrations, they demonstrate a commitment to their roles and a desire to improve their work environment and team performance.
Brian, 51, is a Senior Field Inspector known as “The Motivator,” who works to keep his FDA team engaged and productive. He’s focused on managing his team efficiently but is hampered by outdated software, cumbersome communication processes, and excessive manual paperwork.
Andrew, 34, is a Field Inspector, holds the title “The Traveler”. He values teamwork, and enjoys the travel aspect of his job. Andrew faces obstacles with slow software, connectivity issues, and personal costs for work equipment. He’s proactive in his approach, preparing thoroughly for inspections and advocating for less bureaucracy and more practical work processes, including the ability to work offline.
Journey Map
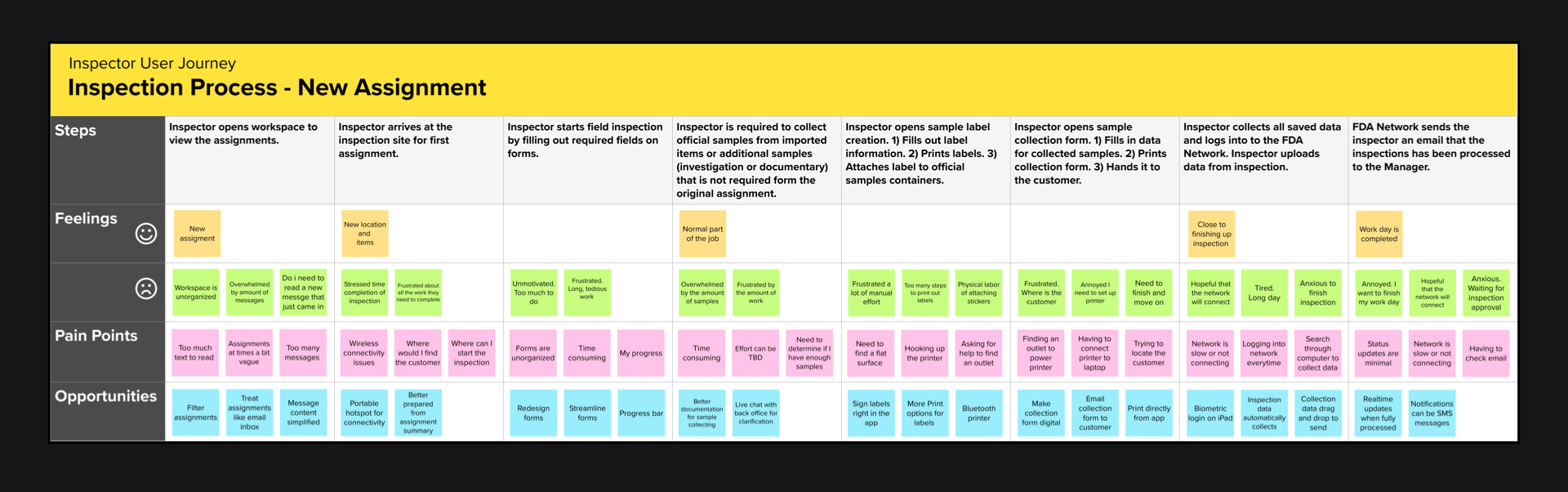
Key Findings
The inspector’s experience points to broader challenges within the FDA’s inspection process. By introducing improved technology, simplifying procedures, and ensuring clear communication, we can greatly increase efficiency and make the job more satisfactory for inspectors.
Solving these problems would not only make inspectors more effective but also enhance the overall quality of public health services. The insights from the inspectors’ daily experiences should be used to push for practical changes and improvements in the system.
Research
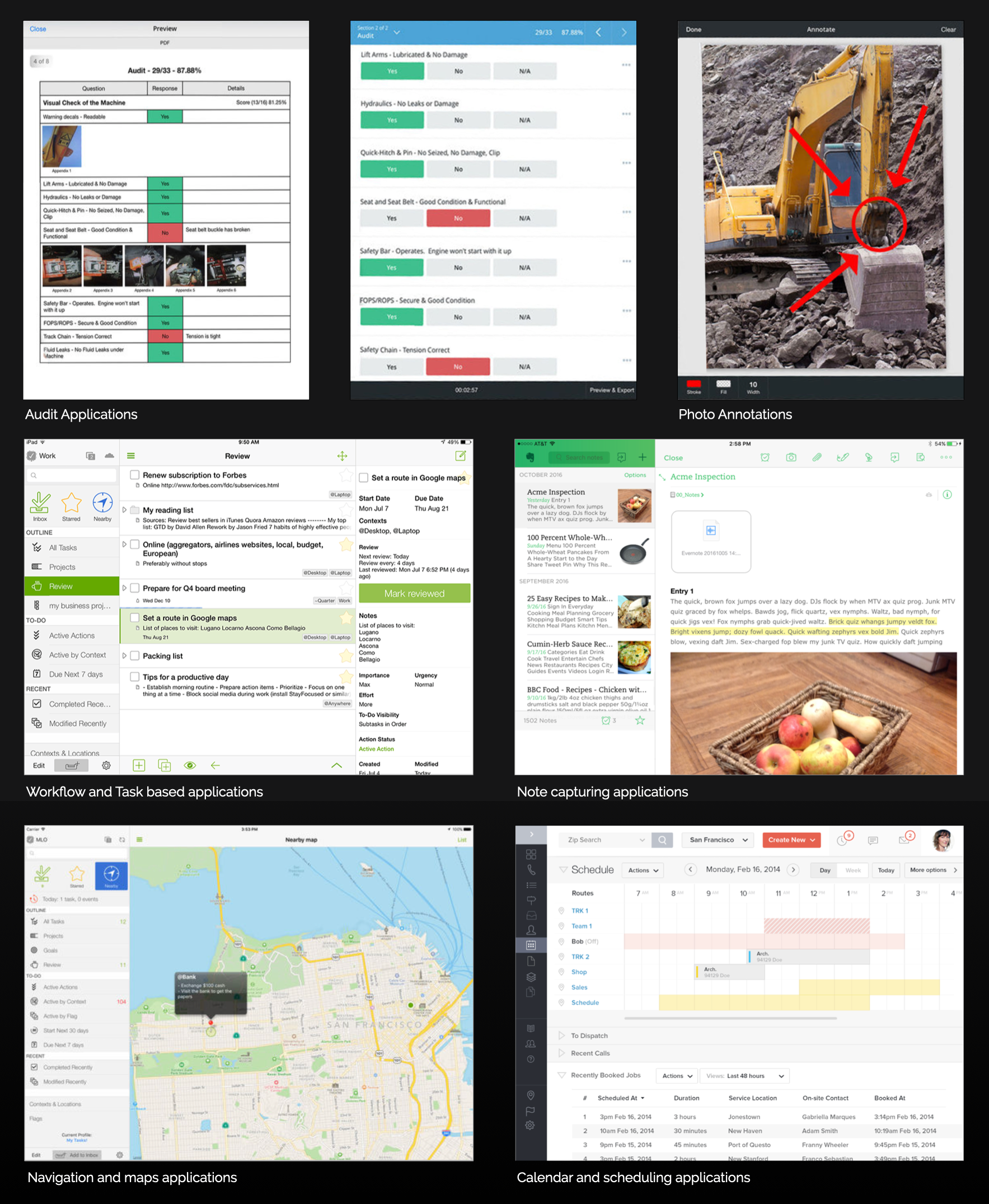
STAGE 2: CONCEPTUALIZE – Key Findings Overview
To enhance the efficiency and effectiveness of operations, it’s crucial to focus on optimizing logistical planning, embracing process automation, updating technological tools, managing personal equipment expenses, and improving team dynamics.
These strategies aim to resolve current frustrations and challenges, leading to a more streamlined, productive, and collaborative work environment.
STAGE 3: DESIGN
Tasks
- Develop Prototype
- Set Up User Testing Frameworks
- Craft High-Fidelity Designs
- Establish a Comprehensive Design System
Prototype & User Testing
Key Findings
- Updated more focused global navigation with adequate options for easy access to key sections.
- Refined digital forms and progress indicator for smoother page-to-page transitions.
- Clear distinction of content and text field states (active, inactive, prefilled, etc) for enhanced page legibility.
- Recommendations for larger interaction areas and a preference for voice-to-text functionality, facilitating hands-free use.
STAGE 3 DESIGN – Key Findings Overview
- Portability & User-Friendly Design: Lightweight and portable with an intuitive, more efficient interface for enhanced usability during inspections.
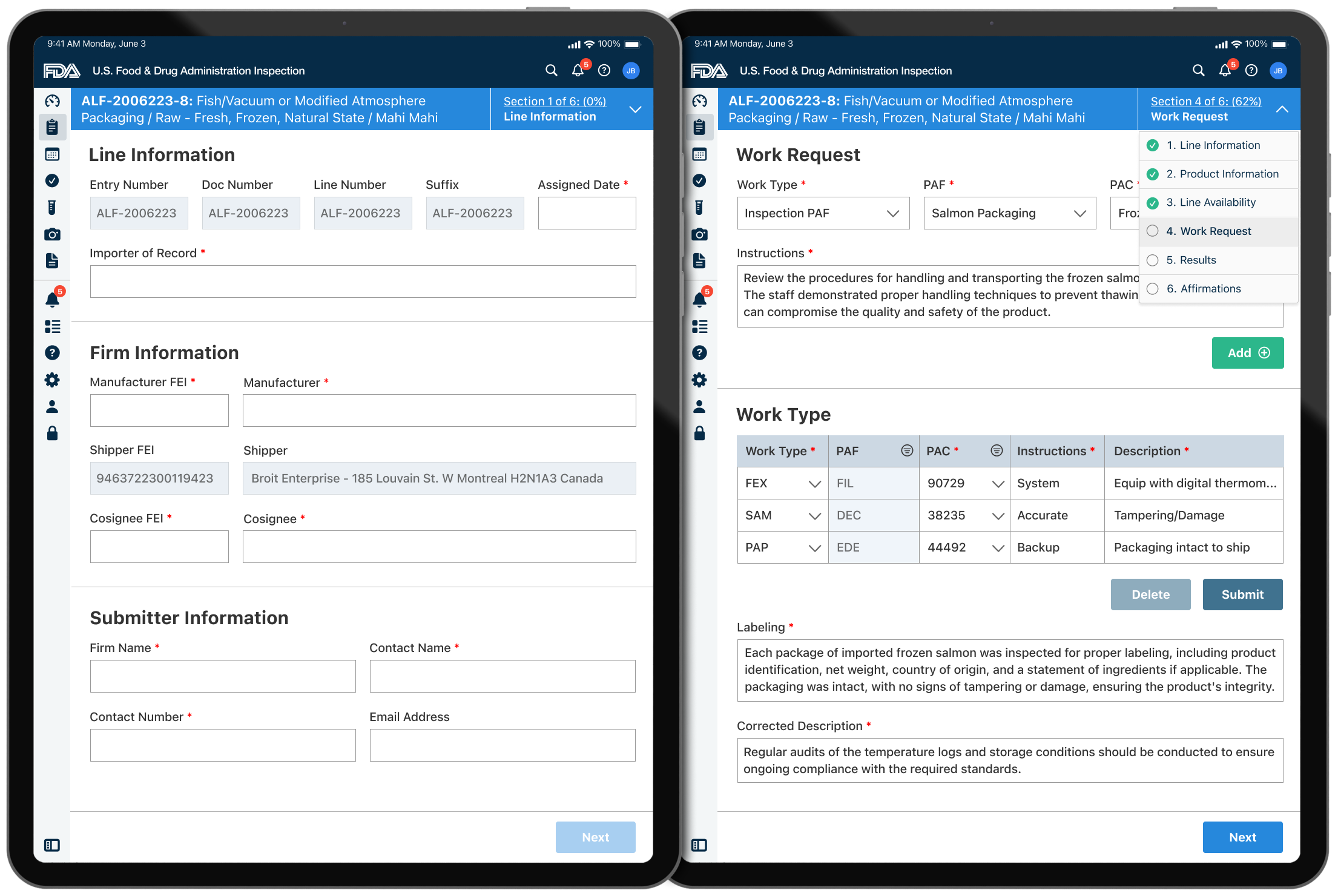
- Data Collection and Reporting: Streamline the process of recording observations and filling out reports.
- Digital Forms and Checklists: Improved efficiency and accuracy with standardized protocols.
- Database Access: Easily store and retrieve inspection reports and compliance records.
- Real-time Communication: Enabled immediate communication with colleagues and superiors.
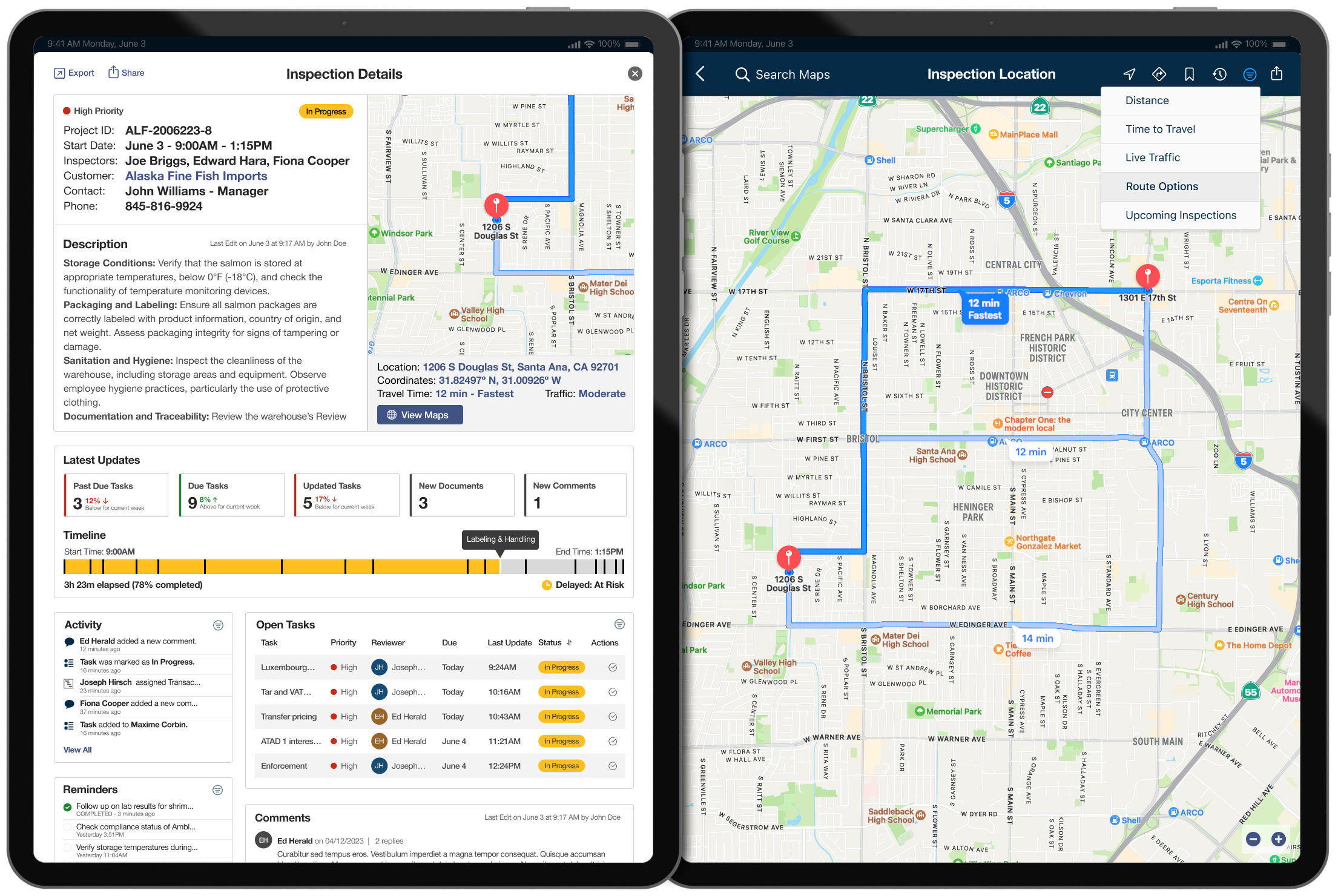
- GPS and Mapping: Assists in planning travel and navigating to inspection sites.
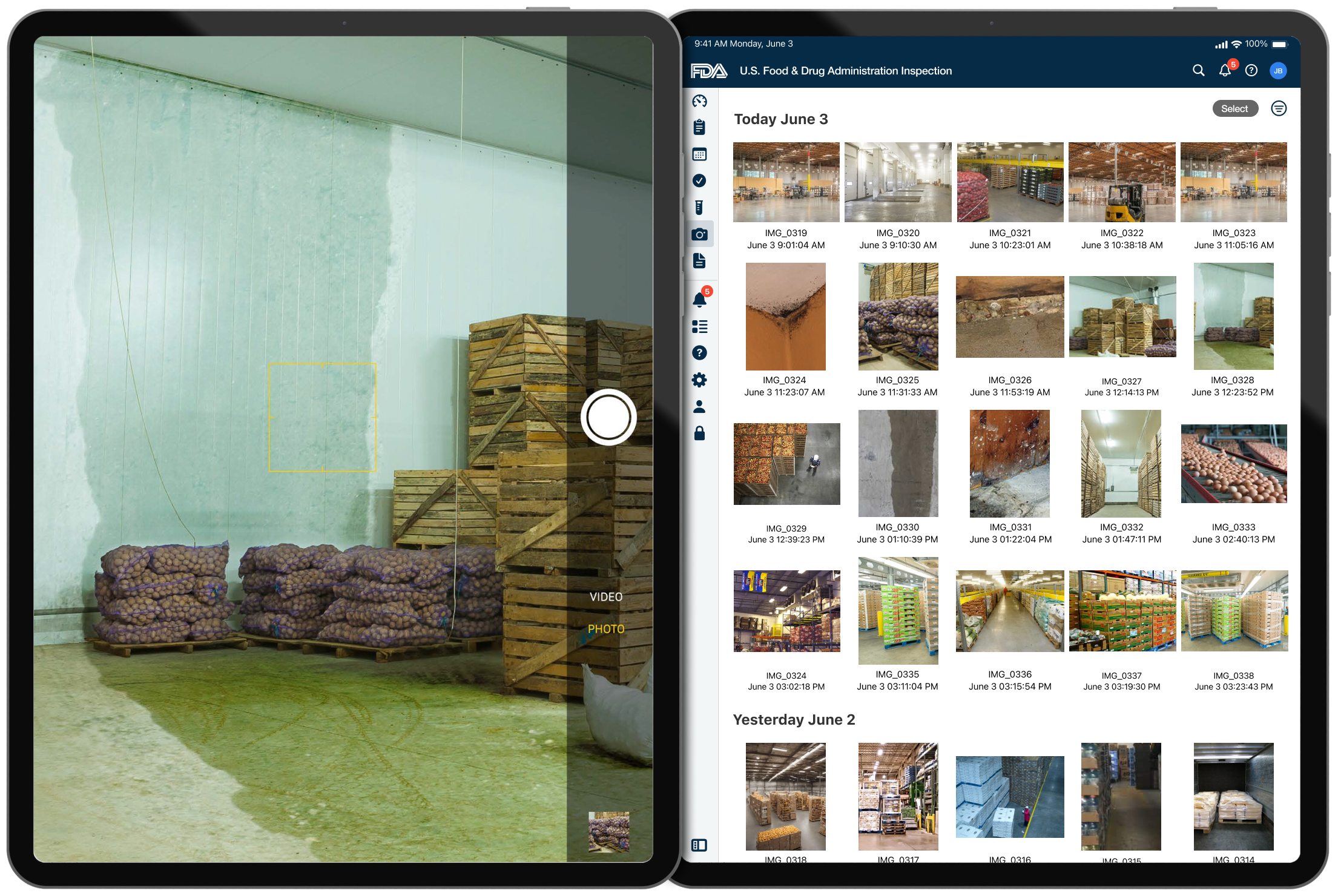
- Camera Functionality: Useful for documenting violations and conditions.
Outcomes
The Company
- Increased Efficiency: Reduced the average time spent on each inspection by 27% due to streamlined data entry and instant access to information.
- Improved Accuracy: Error rates in reporting decreased by 39% due to the app’s guided data entry and validation checks.
- Cost Savings: Saved 18% annually on operational costs, attributed to reduced paper use, improved route planning, and decreased follow-up inspections.
- Positive Feedback: 84% of inspectors reported a significant improvement in job satisfaction and ease of completing inspections.
The Field Inspectors
- Time Management: Inspectors were able to conduct 22% more inspections per week, thanks to efficient data collection and reporting features.
- Quality of Work: 52% reduction in missed violations, ensuring higher compliance rates and safer food practices.
- User Satisfaction: 82% of inspectors found the app more intuitive and easier to use than previous methods, reducing stress and cognitive load.
Knowledge Gained
- Simplified Data Entry: Critical due to extensive reporting needs; achieved through intuitive forms and voice-to-text.
- Instant Access to Information: Integration of searchable databases for regulations and guidelines.
- Customization and Workflow Flexibility: Allowing inspectors to tailor the app to their specific needs.
- Iterative Design & Accessibility: Continuously evolving based on feedback to ensure a robust, user-friendly interface for all environments both indoors and outdoors.
- Real-time Communication: Incorporation of messaging for immediate team updates and queries.
- Navigation and Planning: Use of GPS and mapping for planning and site location.
- Camera Integration: Vital for documenting violations, necessitating easy capture and attachment of images.
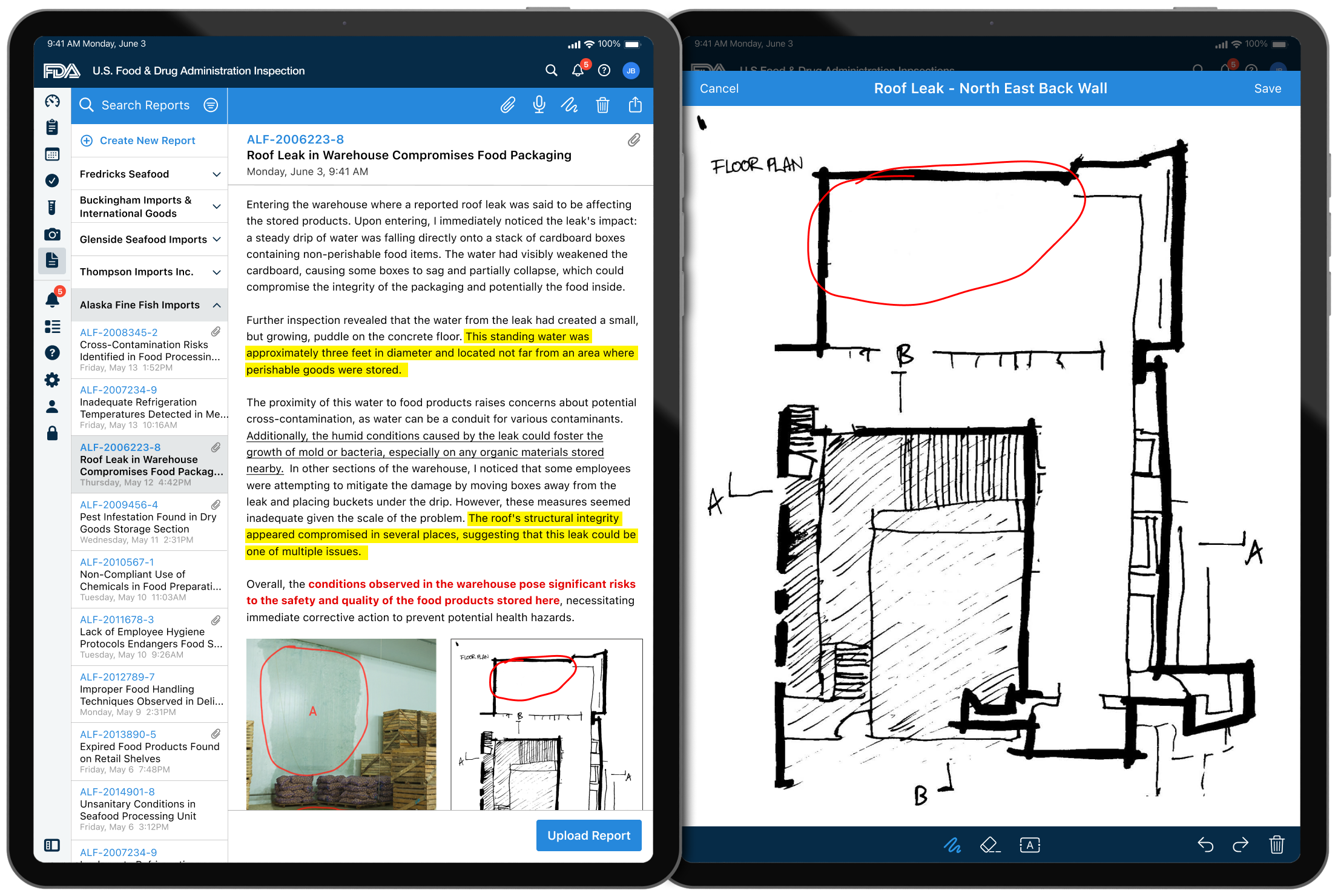
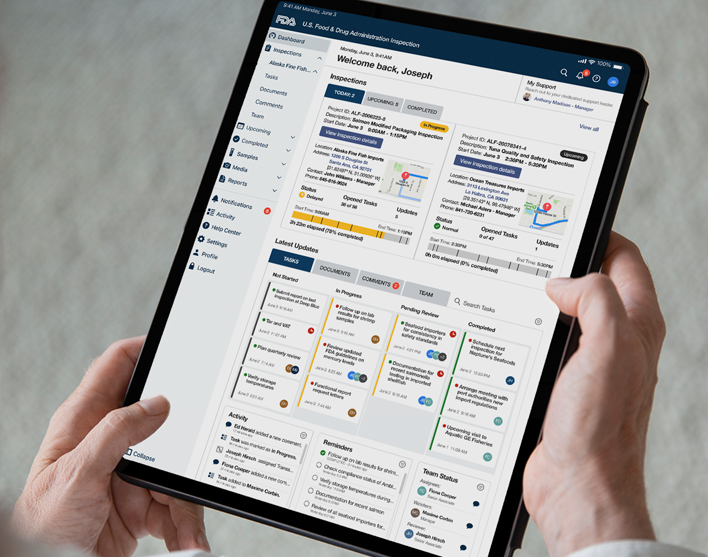
Final Designs
Inspection Dashboard
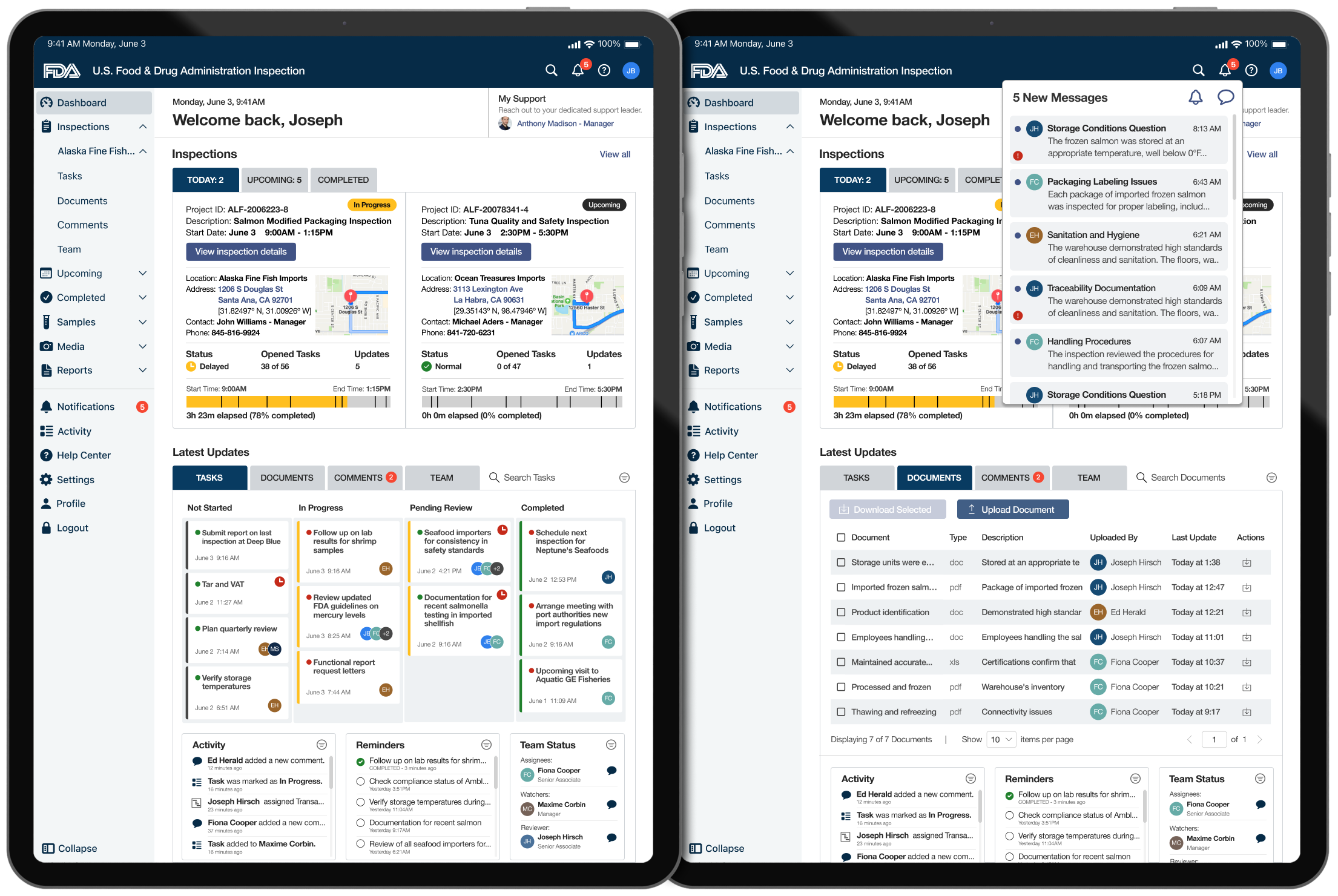
Inspection Dashboard
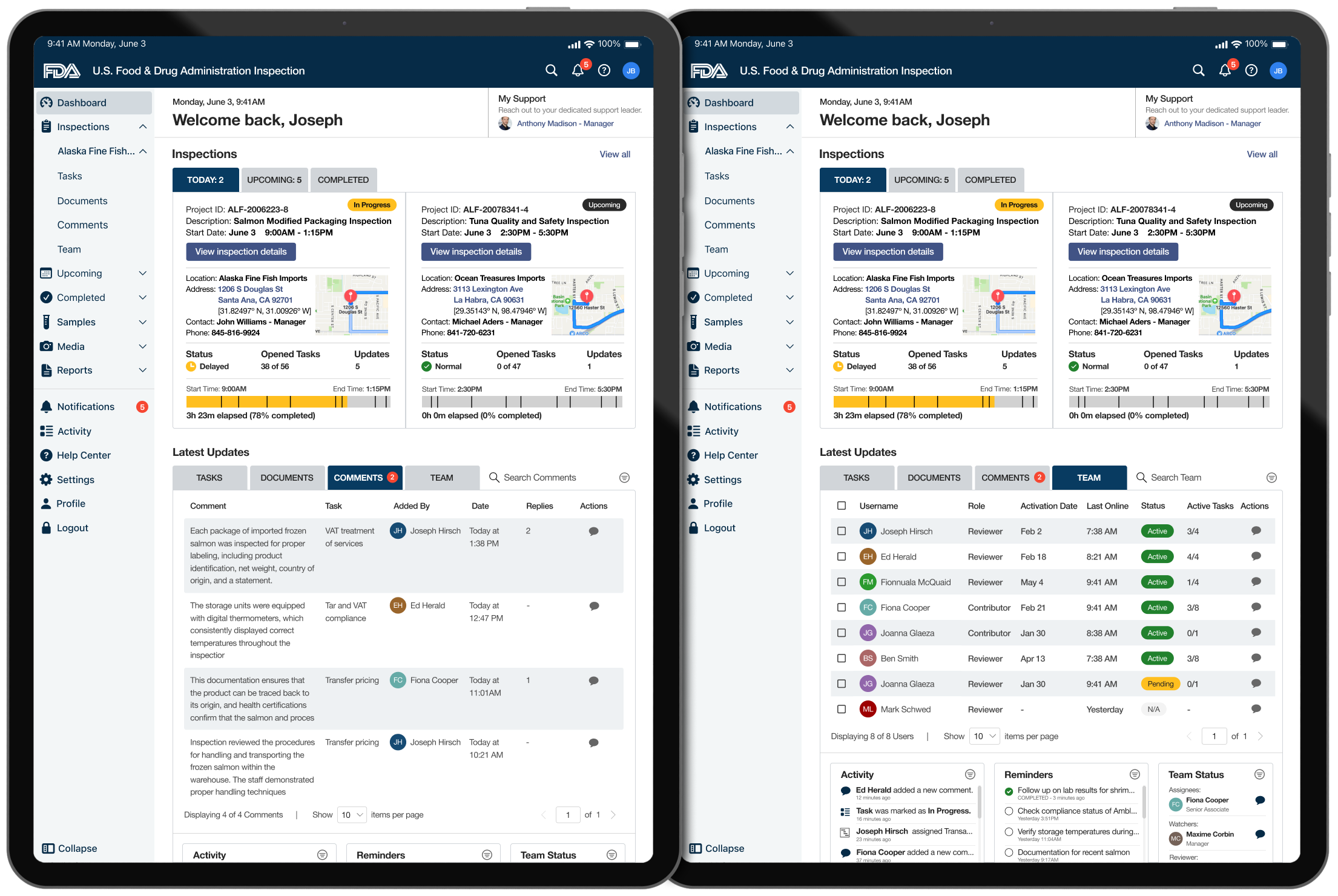
Inspection Details & Location
Inspection Forms
Inspection Sample Collection & Tracking Labels

Inspection Visual Documentation – Collection
Inspection Visual Documentation – Annotations & Markup

Inspection Reports & Markup